THine and Keyssa launch a Detachable HD Camera System Solution
Keyssa and THine
Provides Designers a quick solution for high definition vision systems requiring a ruggedized, low latency, detachable camera.
Combination of different technologies allows breakthrough for emerging industries
TOKYO, JAPAN (January 03, 2019), SANTA CLARA, CA, and CAMPBELL, CA, (January 02, 2019) – THine®, the leader in high-speed serial interfaces and image signal processing, and Keyssa®, the leader in high-speed, contactless connectivity, today announced a HD Camera Proof of Concept solution for ruggedized detachable HD cameras. The detached HD camera enclosure can be water and/or oil proof to accommodate severe outdoor environment conditions.
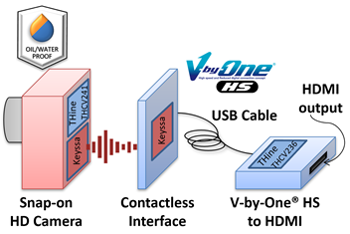
Detachable HD Camera Proof of Concept
Higher resolution cameras are critical to accurate operation and great user experience. Our unique detachable HD camera solution solves the following problems required by advanced vision systems:
Provides high resolution, high frame rate, and low latency to capture and display an accurate image
Performs under severe environments including those requiring water and oil-proof enclosures
Delivers quick video streaming immediately after “snap-on” connection of the rugged detachable camera
The cable-less connection to the camera provides a safer environment for wearable applications by avoiding accidents from cables getting interlaced with clothing or nearby objects
The Proof of Concept solution uses one of THine’s newest chipsets with a MIPI CSI-2 interface, which is a common output of high-resolution cameras, and outputs V-by-One® HS video. The V-by-One® HS protocol is a standard developed by THine Electronics for video transmission up to 4 Gbits/second per lane for greater than 10 meters. It uses a proprietary encoding scheme along with a clock data recovery- (CDR-) based serializer/deserializer (SERDES) technology. A single Keyssa chip wirelessly transmits V-by-One® HS with 60-GHz technology over about a centimeter, which penetrates plastics. The system can operate with no compression-induced delay due to the highspeed signal transfer capability of the Keyssa and THine technologies.
The Proof of Concept shows that the combination of these different technologies from Keyssa and THine will achieve these demanding requirements. At CES 2019, Keyssa will have a private suite to demonstrate many unique solutions including this Proof of Concept.
“The impact will be significant when you think about the numbers of potential use cases for this solution. The gaming and AR industry will have more than 12 million people that could use such devices. Endo/laparoscopic surgery takes place more than 15 million times a year in the US,” said Tak Iizuka, Chief Solution Architect of THine.
Also, by enabling quick and smooth video streaming initialization, the Proof of Concept allows unique system architectures; such as multiple Snap-on Cameras with one Contactless Interface, and one Snap-on Cameras with multiple Contactless Interfaces. Applications include, but are not limited to, wearable/body-top devices for XR (AR/VR/MR), smartphones, tablet-type or lap-top computers, surveillance cameras, home/business security cameras, machine vision, document scanners, medical equipment, and automotive devices.
“THine’s V-by-One® has become a de facto standard for delivering HD video content in consumer and commercial applications,” said Eric Almgren, CEO of Keyssa. “Our wireless connector works seamlessly with THine’s technology to enable new and innovative product designs that require HD video streaming.”
“We are very excited to introduce an unprecedented solution where V-by-One® HS goes over the air thanks to Keyssa’s unique technology,” said Yasuhiro Takada, President and CEO of THine, and “We believe people from many markets will be very impressed with the potential of our state of the art solutions.”